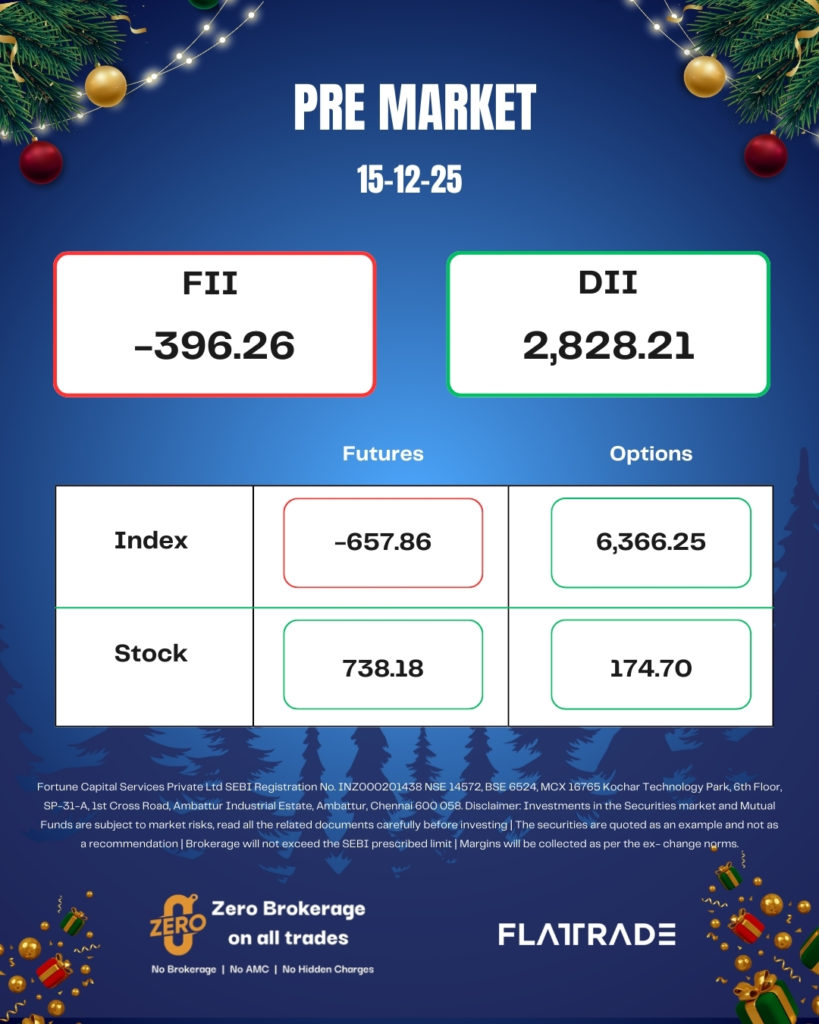
PRE MARKET
Gift Nifty indicates a negative start for the broader index in India, with a loss of 76.5 points or 0.29 percent. The Nifty futures were trading around the 26,058.50 level.
The S&P 500 and the Nasdaq closed down more than 1% on Friday, with investors leaving technology for other sectors as Broadcom and Oracle fueled concerns about an AI bubble, and rising U.S. Treasury yields added pressure after some policymakers spoke out against easing monetary policy.
The Nasdaq Composite lost 398.69 points, or 1.69%, to 23,195.17 while the S&P 500 lost 73.59 points, or 1.07%, to 6,827.41. For the week, the S&P 500 fell 0.63% while the Nasdaq declined 1.62%. The Dow Jones Industrial Average fell 245.96 points, or 0.51% on the day to 48,458.05, but managed to show a weekly gain of 1.05%.
Asian markets opened lower in the final full trading week of 2025 as mounting concerns over the earnings outlook for technology companies, and their massive spending on AI, sapped risk sentiment.
Japan’s Nikkei, Hong Kong’s Hang Seng, and the Taiwanese weighted went down -1.38, -1.00, and -1.01 percent, respectively.
STOCKS TODAY
Wipro
The AI-powered technology services and consulting company has expanded its longstanding partnership with Google Cloud to enhance enterprise productivity and drive global digital transformation with Gemini Enterprise. Additionally, the company announced a three-year strategic partnership with Microsoft to help enterprises transform into Frontier Firms, early leaders in AI adoption.
Dr Reddy’s Laboratories
The United States Food & Drug Administration (USFDA) completed a GMP and a Pre-Approval Inspection (PAI) at the company’s formulations facility (FTO-SEZ PU01) in Srikakulam, Andhra Pradesh, and issued a Form 483 with five observations. The inspection was conducted from December 4–12.
SMS Pharmaceuticals
The United States Food and Drug Administration (USFDA) inspected the company’s Active Pharmaceutical Ingredient (API) manufacturing facility at Visakhapatnam, Andhra Pradesh, during December 8–12, and concluded with one minor observation in Form 483. The observation is procedural in nature and does not relate to data integrity.
Aurobindo Pharma
The United States Food and Drug Administration (USFDA) inspected Unit-V, an API manufacturing facility of the company’s subsidiary Apitoria Pharma, in Telangana during December 1–12, and concluded with the issuance of a Form 483 with three observations, which are procedural in nature.
Bharat Electronics
The company has bagged additional orders worth Rs 776 crore since November 14, including indigenous counter-unmanned aerial systems (SAKSHAM), software-defined radios, anti-drone systems, communication equipment, medical electronics, gun control systems, weapon control systems, security software, avionics, masts, components, upgrades, spares, and services.
Source – Moneycontrol