PRE MARKET
Gift Nifty indicates a positive start for the broader index in India, with a gain of 52 points or 0.21 percent. The Nifty futures were trading around the 24,791.50 level.
Wall Street ended sharply lower on Friday after Iran launched missiles at Israel in response to intensive Israeli strikes aimed at crippling Tehran’s ability to build nuclear weapons.
The S&P 500 declined 1.13% to end the session at 5,976.97 points. The Nasdaq fell 1.30% to 19,406.83 points, while the Dow Jones Industrial Average dropped 1.79% to 42,197.79 points.
Asian marktradedding mostly higher on Monday, as investors assessed escalating Israel-Iran tensons, while awaiting a slew of data from China.
Japan’s Nikkei went up 0.96 percent, KOSPI Index also went up 0.59 percent, while Hong Kong’s Hang Seng and the Taiwanese index decreased a mere 0.05 and 0.37 percent, respectively.
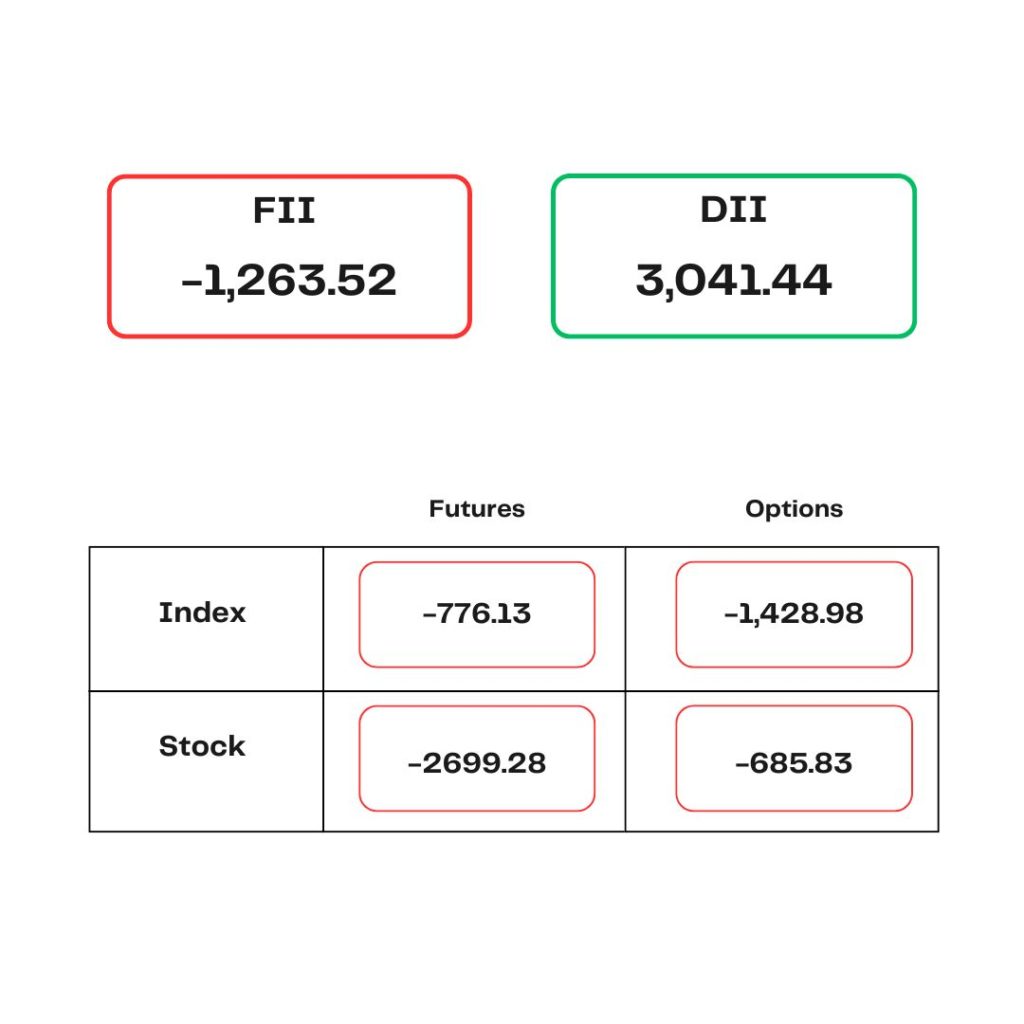
STOCKS TODAY
Natco Pharma
The USFDA concluded its inspection of the company’s API manufacturing plant in Hyderabad, conducted during June 9–13, and issued a Form 483 with one observation. The company stated that the observation is procedural and expressed confidence in addressing it comprehensively.
South Indian Bank
Biju E Punnachalil, General Manager, Chief Risk Officer, and Key Managerial Personnel of the bank, has opted for voluntary retirement to pursue other opportunities. He will be retiring from the bank effective July 11.
Godrej Properties
The Mumbai-based real estate developer will develop a premium residential project on a 14-acre land parcel in Hoskote, Bengaluru. The proposed development is expected to offer approximately 1.5 million square feet of saleable area, with an estimated revenue potential of Rs 1,500 crore.
Sun Pharmaceutical Industries
The US Food and Drug Administration (USFDA) conducted a Good Manufacturing Practices (GMP) inspection of the company’s Halol facility in Gujarat during June 2–13. After the inspection, the USFDA issued a Form 483 with eight observations. Additionally, the Board has appointed Kirti Ganorkar as the company’s Managing Director for five years, effective September 1, 2025. Dilip Shanghvi will continue as the Executive Chairman of the company’s Board.
Syngene International
The USFDA conducted a Good Clinical Practices (GCP) compliance inspection of the company’s facility at Semicon Park, Bengaluru, during June 9–13. The inspection concluded with zero observations, and no Form 483 was issued. The inspection has been classified as No Action Indicated (NAI). Separately, the company received an Establishment Inspection Report (EIR) from the USFDA for its manufacturing facilities at Biocon Park, SEZ, Bengaluru. The EIR concluded the inspectional outcome as Voluntary Action Indicated (VAI).
Source – Moneycontrol