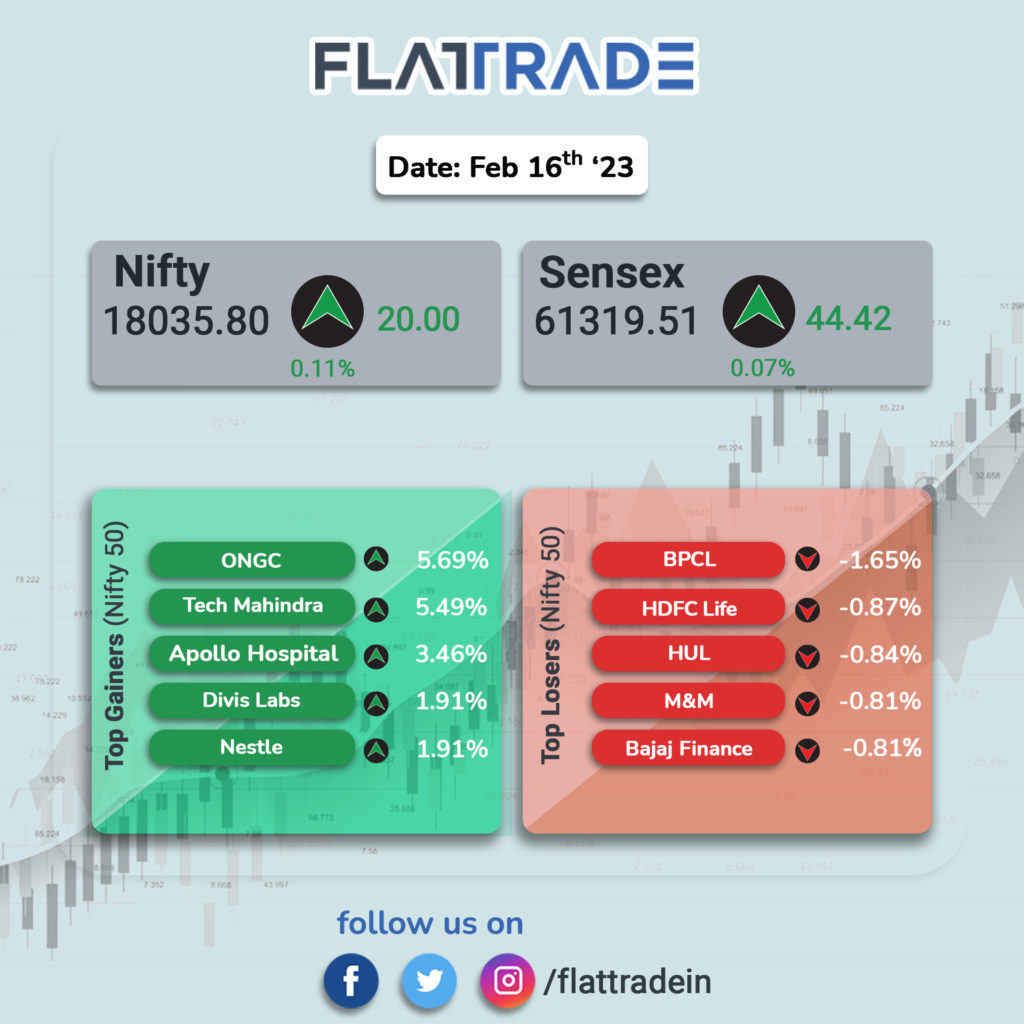
Benchmark stock indices ends flat amid volatility; realty, IT, metals shine. The BSE midcap and smallcap added nearly a percent each. The Sensex ends flat at 0.07% and the Nifty 50 index was up 0.11%.
Among sectors, information technology, metal and Realty up 1 percent each.
ONGC, Tech Mahindra, Apollo Hospitals, Divis Labs and Nestle India among the major gainers on the Nifty, while losers were BPCL, Bajaj Finance, HUL, HDFC Life and M&M.
Indian rupee down by 8 paise to 82.71 against the US dollar on Thursday.
Stock in News Today
Nestle India: The FMCG giant posted a 62 percent year-on-year jump in its net profit at Rs 628 crore for the October-December 2022 quarter.Net profit stood at Rs 386 crore in the same period last year, after an impact of Rs 236.5 crore exceptional loss. Operating margins improved to 23 percent from 22.8 percent YoY.
Adani Power: The power company has called off its plan to acquire a coal plant project in central India, as billionaire Gautam Adani looks to rein in spending and rebuild investor confidence in the wake of a bruising short seller report, Bloomberg reported
Bharat Electronics: The company has signed an MoU with Israel Aerospace Industries (IAI) for the domestic manufacture and supply of its LORA Weapon System for Indian Triservices. The state-of-the-art strategic weapon system weapon will be manufactured by BEL, as the prime contractor, based on the workshare arrangement with IAI.
PI Industries: Agrochemical company PI Industries has reported a strong Q3FY23. net profit for the quarter stood at Rs 352 crore, beating street estimates and driving a surge in its stock price. Its stock went up by more than 9 percent intraday on February 16.
Bharat Forge: Bharat Forge and Paramount Group signed a Memorandum of Understanding (MoU) to develop and produce Composite Rotor Blades, Mission Systems, and Stores Management systems for Medium Lift Helicopters at Aero India today.
Shilpa Medicare: Its Bengaluru facility for manufacturing and testing Orodispersible films and Transdermal systems has been successfully registered with the Ministry of Health, United Arab Emirates. This registration enables the company to register the products (Orodispersible films and Transdermal systems) in UAE for commercialization.
Piramal Pharma: USFDA has issued an Establishment Inspection Report (EIR) for the manufacturing facility of Piramal Pharma located at Lexington (Kentucky, USA). The inspection has now been successfully closed by the US FDA.



